Research Group - Prof. Thorsten Klüner
Electronic structure calculations of oxidation reactions on nanoporous gold
The oxidation of methanol in the gas phase, in aqueous solution, and under electrocatalytic conditions are three types of catalytic processes taking place in different reaction environments and with different target practical applications but sharing many of the common mechanistic principles in the transformations of molecules. Our Research Unit brings these areas together by studying nanoporous gold (npAu) as a common catalytic material, focusing on the interplay between composition and structural parameters and the catalytic properties.
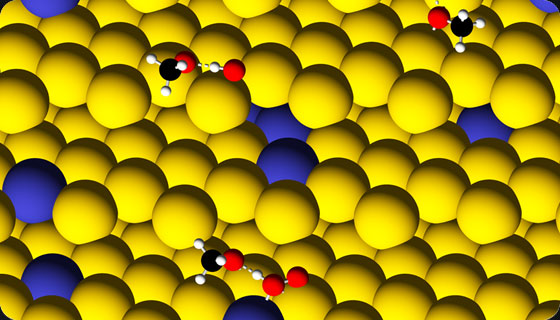
Whereas the CH3OH oxidation in the gas phase or in the liquid phase over npAu are partial oxidation reactions converting methanol to methyl formate or to formic acid, respectively,[1] the electrocatalytic oxidation of methanol on a gold anode leads to its full oxidation to CO, CO2 and H2O. The selective oxidation of alcohols to carbonyl compounds is an important step in the synthesis of fine chemicals.[2] To this end, aerobic oxidation using gold-based catalysts offers a sustainable, environmentally friendly alternative to currently used synthetic methods. The electrocatalytic oxidation is of potential interest for direct conversion of methanol to electricity in fuel cells. The interest to npAu as an electrode material arises from the lack of electrode poisoning by-products on Au, such as CO, and lower material costs compared to conventional Pt-based electrocatalysts.[3]
The water-gold interface provides a reaction environment that enhances the catalytic performance of nanostructured gold. In aqueous medium, especially under alkaline conditions, gold nanocrystals were found to be highly effective for the oxidation of alcohols in the presence of O2.[4] Recent studies pointed to the importance of oxo and/or hydroxo species on the investigated Au surfaces for the observed catalytic activity.[5] In the gas-phase studies water was proposed to help bind and activate molecular oxygen on nanostructured Au catalysts.[6] At variance to generally inert single-crystal Au surfaces, npAu appears to inherently contain on-surface and subsurface oxygen atoms, which may penetrate deep in the material during its conventional preparation via dealloying.[7] Contact with water probably modifies the surface composition creating OH groups. The surface composition is most likely strongly pH-dependent. Surface corrugation/roughness is also believed to be the key factors responsible for the catalytic activity of npAu. Understanding the role of (i) surface morphology and, in particular, low-coordinated sites, (ii) lattice strain through the surface of nanoporous gold, (iii) concentration and distribution of an impurity metal, such as Ag or Pt, (iv) surface population of oxygenates, e.g. O2, O, OH, OOH, and H2O, in the complicated network of elementary reactions is the major objective of the current sub-project. Such an insight at the atomistic level will be provided by first-principles theoretical studies.
We will use two general types of computational approaches: periodic slab models with periodic boundary conditions and finite embedded cluster models. Large part of calculations will be based on density functional theory (DFT) and will employ a periodic supercell approach.[11] For selected critical reaction steps benchmark calculations with a recently proposed self-consistent embedded cluster approach [46] will be carried out.
References
[1] a) A. Wittstock, V. Zielasek, J. Biener, C. M. Friend, M. Bäumer, Science 2010, 327, 319-322;
b) B. N. Zope, D. D. Hibbitts, M. Neurock, R. J. Davis, Science 2010, 330, 74-78.
[2] R. A. Sheldon, I. W. C. E. Arends, G.-J. ten Brink, A. Dijksman, Acc. Chem. Res. 2002, 35, 774-781.
[3] a) J. Wang, R. Shi, X. Guo, J. Xi, J. Zhao, C. Song, L. Wang, J. Zhang, Electrochim. Acta 2014, 123, 309-316.
b) M. Yin, Y. Huang, L. Liang, J. Liao, C. Liu, W. Xing, Chem. Commun. 2011, 47, 8172-8174.
[4] A. Abad, C. Almela, A. Corma, H. García, Tetrahedron 2006, 62, 6666-6672.
[5] O. Diaz-Morales, F. Calle-Vallejo, C. de Munck, M. T. M. Koper, Chem. Sci. 2013, 4, 2334-2343.
[6] M. Daté, M. Okumura, S. Tsubota, M. Haruta, Angew. Chem. Int. Ed. 2004, 43, 2129-2132.
[7] S. Röhe, K. Frank, A. Schaefer, A. Wittstock, V. Zielasek, A. Rosenauer, M. Bäumer, Surf. Sci. 2013, 609, 106-112.

Prof. Thorsten Klüner
UNIVERSITÄT OLDENBURG
PHYSIKALISCHE CHEMIE
Email: thorsten.kluener@uni-oldenburg.de
Phone: 0441-7983681
Niklas Thoben, PhD student
Contact
Spokeperson:
Prof. Gunther Wittstock
Deputy Spokeperson:
Prof. Marcus Bäumer
Administrative Contact:
Prof. Gunther Wittstock

